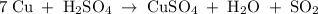Chemistry, 13.01.2020 01:31 rodfam13716
7. copper + sulfuric acid yield copper (ii) sulfate + water + sulfur dioxide

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

You know the right answer?
7. copper + sulfuric acid yield copper (ii) sulfate + water + sulfur dioxide...
Questions

English, 24.01.2021 14:00


English, 24.01.2021 14:00



Law, 24.01.2021 14:00

Mathematics, 24.01.2021 14:00



Social Studies, 24.01.2021 14:00

Social Studies, 24.01.2021 14:00

Mathematics, 24.01.2021 14:00

Arts, 24.01.2021 14:00

English, 24.01.2021 14:00




Chemistry, 24.01.2021 14:00