Given 24.3 g of lici, find moles of pbci, produced.
pbso4 + licl → pbcl2 + li2so4
...

Chemistry, 13.01.2020 07:31 jodygoodwin40
Given 24.3 g of lici, find moles of pbci, produced.
pbso4 + licl → pbcl2 + li2so4
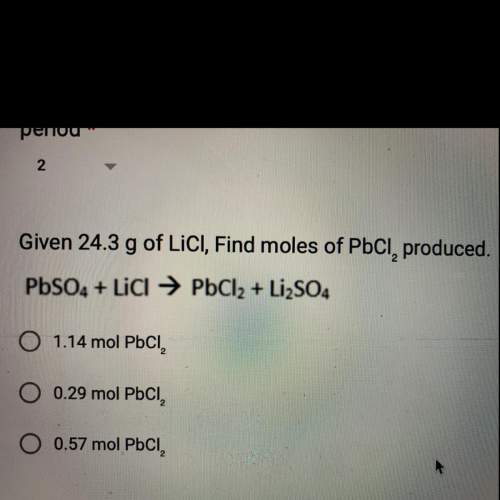

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1
You know the right answer?
Questions

Mathematics, 11.08.2021 15:10

Medicine, 11.08.2021 15:10

Biology, 11.08.2021 15:10

Computers and Technology, 11.08.2021 15:10

Biology, 11.08.2021 15:10

Social Studies, 11.08.2021 15:20

Biology, 11.08.2021 15:20

Social Studies, 11.08.2021 15:20

Mathematics, 11.08.2021 15:20

Mathematics, 11.08.2021 15:20

Mathematics, 11.08.2021 15:20

Biology, 11.08.2021 15:20

Social Studies, 11.08.2021 15:20

Mathematics, 11.08.2021 15:20

English, 11.08.2021 15:20

Mathematics, 11.08.2021 15:20


English, 11.08.2021 15:20


Mathematics, 11.08.2021 15:20



