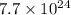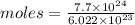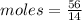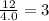Chemistry, 13.01.2020 09:31 leopolesamoy
Aparticular compound in the chemistry laboratory is found to contain 7.2x10^24 atoms of oxygen, 56.0g of nitrogen, and 4.0 mol of hydrogen. what is it’s empirical formula?


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
Aparticular compound in the chemistry laboratory is found to contain 7.2x10^24 atoms of oxygen, 56.0...
Questions


Advanced Placement (AP), 07.10.2020 23:01






Mathematics, 07.10.2020 23:01




Computers and Technology, 07.10.2020 23:01


History, 07.10.2020 23:01


Biology, 07.10.2020 23:01



History, 07.10.2020 23:01

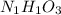
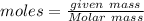
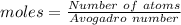
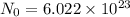 atoms
atoms