Chemistry, 13.01.2020 19:31 nayelimoormann
A0.483-g sample of nonanedioic acid (c9h16o4) is burned in a bomb calorimeter and the temperature increases from 24.70 °c to 27.20 °c. the calorimeter contains 1.01×103 g water and the bomb has a heat capacity of 867 j/°c. based on this experiment, calculate δe (kj/mol) for the combustion reaction.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 06:30
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3
You know the right answer?
A0.483-g sample of nonanedioic acid (c9h16o4) is burned in a bomb calorimeter and the temperature in...
Questions


Chemistry, 09.03.2021 02:10

Mathematics, 09.03.2021 02:10


Mathematics, 09.03.2021 02:10



Mathematics, 09.03.2021 02:10


Biology, 09.03.2021 02:10


Chemistry, 09.03.2021 02:10





Mathematics, 09.03.2021 02:10

Mathematics, 09.03.2021 02:10


Mathematics, 09.03.2021 02:10

![q=[q_1+q_2]](/tpl/images/0453/1616/341bc.png)
![-q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0453/1616/37582.png)
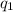 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter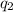 = heat absorbed by the water
= heat absorbed by the water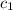 = specific heat of calorimeter =
= specific heat of calorimeter = 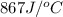
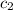 = specific heat of water =
= specific heat of water = 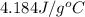
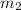 = mass of water =
= mass of water = 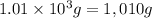
 = change in temperature = 27.20 °C - 24.70 °C =2.5°C
= change in temperature = 27.20 °C - 24.70 °C =2.5°C![-q=[(867 J/^oC\times 2.5 ^oC)+(1,010\times 4.184J/g^oC\times 2.5^oC)]](/tpl/images/0453/1616/a0608.png)
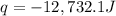
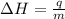
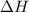 = enthalpy change = ?
= enthalpy change = ?