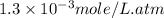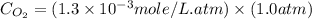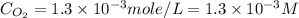Chemistry, 13.01.2020 21:31 aneisha0117
What is the molar concentration of oxygen in water at 25°c for a partial pressure of 1.0 atm ( = 1.3 x 10⁻³ mol/latm) a. 7.2 x 10⁻⁴b. 1.3 x 10⁻³ m c. 7.7 x 10⁴d. 1.3 x 10⁻⁵
= 1.3 x 10⁻³ mol/latm) a. 7.2 x 10⁻⁴b. 1.3 x 10⁻³ m c. 7.7 x 10⁴d. 1.3 x 10⁻⁵

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
What is the molar concentration of oxygen in water at 25°c for a partial pressure of 1.0 atm ([tex]k...
Questions


Mathematics, 10.03.2021 17:40

Mathematics, 10.03.2021 17:40


Mathematics, 10.03.2021 17:40

Mathematics, 10.03.2021 17:40


Mathematics, 10.03.2021 17:40



Mathematics, 10.03.2021 17:40




History, 10.03.2021 17:40


Mathematics, 10.03.2021 17:40



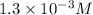
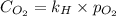
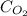 = molar concentration of
= molar concentration of 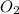 = ?
= ?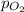 = partial pressure of
= partial pressure of 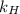 = Henry's law constant =
= Henry's law constant =