rate = k[n_2]^3 [o_3]

Chemistry, 14.01.2020 00:31 helphelp81
The rate of a certain reaction is given by the following rate law:
rate = k[n_2]^3 [o_3]
use this information to answer the questions below.
1. what is the reaction order in n_2?
2. what is the reaction order in o_3?
3. what is overall reaction order?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1
You know the right answer?
The rate of a certain reaction is given by the following rate law:
rate = k[n_2]^3 [o_3]
rate = k[n_2]^3 [o_3]
Questions



Mathematics, 14.10.2021 22:10

Mathematics, 14.10.2021 22:10




English, 14.10.2021 22:10

Mathematics, 14.10.2021 22:10




History, 14.10.2021 22:10







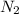 = 3
= 3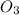 = 1
= 1![rate = k[N_2]^3 [O_3]](/tpl/images/0453/5258/fc831.png)


