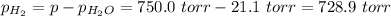Chemistry, 14.01.2020 02:31 steffweikeloyrt00
Asample of h2 gas is collected over water at 23 degrese celsius, if the observed pressure is 750.0 torr, calculate the partial pressure of h2

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
Asample of h2 gas is collected over water at 23 degrese celsius, if the observed pressure is 750.0 t...
Questions

Biology, 22.08.2019 14:50

Social Studies, 22.08.2019 14:50

Mathematics, 22.08.2019 14:50

Mathematics, 22.08.2019 14:50

Mathematics, 22.08.2019 14:50


Physics, 22.08.2019 14:50




Computers and Technology, 22.08.2019 14:50

Mathematics, 22.08.2019 14:50

Biology, 22.08.2019 14:50



Physics, 22.08.2019 14:50

Mathematics, 22.08.2019 14:50


Social Studies, 22.08.2019 14:50