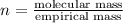Which statement is true about an empirical formula?
-it is the true ratio of atoms in a...

Chemistry, 26.10.2019 00:43 tfyvcu8052
Which statement is true about an empirical formula?
-it is the true ratio of atoms in a formula unit.
-it is the simplest ratio of atoms in a formula unit.
-it represents the true molecular mass of a chemical formula.
-it represents the highest ratio of coefficients in a chemical formula.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
Questions



Mathematics, 25.01.2021 20:30

Mathematics, 25.01.2021 20:30

Computers and Technology, 25.01.2021 20:30



Mathematics, 25.01.2021 20:30

Mathematics, 25.01.2021 20:30




Mathematics, 25.01.2021 20:30





Mathematics, 25.01.2021 20:30

English, 25.01.2021 20:30

English, 25.01.2021 20:30