
Chemistry, 14.01.2020 04:31 elijahjacksonrp6z2o7
Which of the following best to account for the fact that the f- ion is smaller than the o2- ion?
a. f- has a larger nuclear mass than o2- has
b. f- has a larger nuclear charge than o2- has
c. f- has more electrons than o2- has
d. f- is more electronegative than o2- is
e. f- is more polarizable than o2- is

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

You know the right answer?
Which of the following best to account for the fact that the f- ion is smaller than the o2- ion?
Questions


Social Studies, 11.10.2019 03:30



Biology, 11.10.2019 03:30

Mathematics, 11.10.2019 03:30

Mathematics, 11.10.2019 03:30

Spanish, 11.10.2019 03:30

History, 11.10.2019 03:30

History, 11.10.2019 03:30

History, 11.10.2019 03:30

Mathematics, 11.10.2019 03:30


Mathematics, 11.10.2019 03:30


History, 11.10.2019 03:30

Mathematics, 11.10.2019 03:30



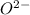 will be larger.
will be larger.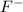 has a larger nuclear charge than
has a larger nuclear charge than 

