Chemistry, 14.01.2020 23:31 morenodonaldo762
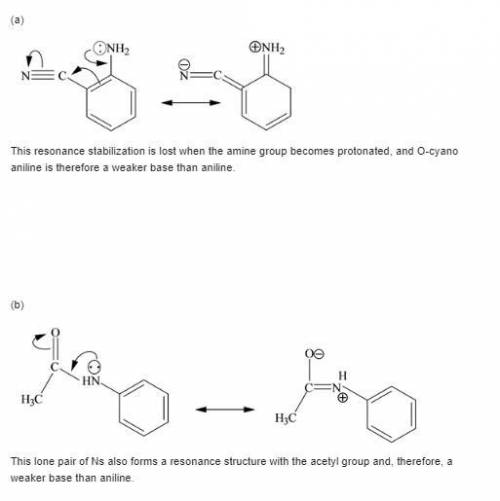
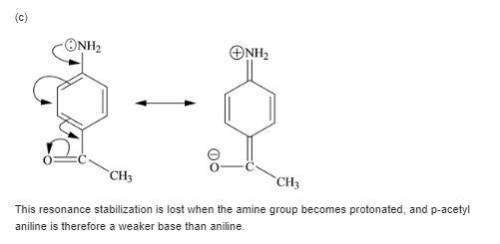
Each of the following is a much weaker base than aniline. present a resonance argument to explain the effect of the substituent in each case.
a. o-cyanoaniiine
b. p-aminoacetophenone
c. c6h5nhcch3
||
o

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1


Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2
You know the right answer?
Each of the following is a much weaker base than aniline. present a resonance argument to explain th...
Questions




Arts, 07.07.2019 08:30


Mathematics, 07.07.2019 08:30

Mathematics, 07.07.2019 08:30


English, 07.07.2019 08:30

Geography, 07.07.2019 08:30


Chemistry, 07.07.2019 08:30


Social Studies, 07.07.2019 08:30

Mathematics, 07.07.2019 08:30


Mathematics, 07.07.2019 08:30

Biology, 07.07.2019 08:30