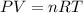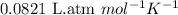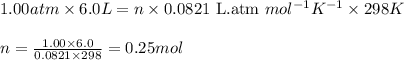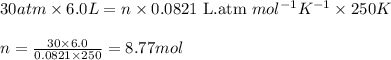The average lung capacity of a human is 6.0l.
how many moles of air are in your lungs, when you are in the following situations?
(a) at sea level where t=298 k, and p = 1.00 atm.
(b) on top of mt everest where t = 200 k and p = 0.296 atm.
(c) trying to escape from a sunken submarine where t = 250 k and p = 30 atm.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
The average lung capacity of a human is 6.0l.
how many moles of air are in your lungs, when yo...
how many moles of air are in your lungs, when yo...
Questions

Spanish, 06.12.2019 02:31

Physics, 06.12.2019 02:31


Health, 06.12.2019 02:31

History, 06.12.2019 02:31


Mathematics, 06.12.2019 02:31

Geography, 06.12.2019 02:31


English, 06.12.2019 02:31

English, 06.12.2019 02:31

Arts, 06.12.2019 02:31

Business, 06.12.2019 02:31

Mathematics, 06.12.2019 02:31


Mathematics, 06.12.2019 02:31

History, 06.12.2019 02:31


Mathematics, 06.12.2019 02:31

Mathematics, 06.12.2019 02:31