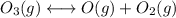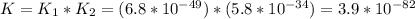Consider the following data. no2(g) equilibrium reaction arrow identifying the requirement for light no(g) + o(g) k = 6.8 ✕ 10-49 o3(g) + no(g) equilibrium reaction arrow no2(g) + o2(g) k = 5.8 ✕ 10-34 calculate a value for the equilibrium constant for the reaction below. (hint: when reactions are added together, the equilibrium expressions are multiplied.)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
Consider the following data. no2(g) equilibrium reaction arrow identifying the requirement for light...
Questions


Law, 17.08.2021 16:20

World Languages, 17.08.2021 16:20


Social Studies, 17.08.2021 16:30





Mathematics, 17.08.2021 16:30


Mathematics, 17.08.2021 16:30


Mathematics, 17.08.2021 16:30


Computers and Technology, 17.08.2021 16:30


Social Studies, 17.08.2021 16:30


Mathematics, 17.08.2021 16:30

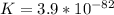

![K_1=\frac{[NO][O]}{[NO_2]}=6.8*10^{-49}](/tpl/images/0455/4423/c0bdd.png)

![K_2=\frac{[NO_2}{[O_3][NO]}=5.8*10^{-34}](/tpl/images/0455/4423/ae794.png)