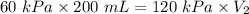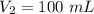The pressure on a 200-milliliter sample of co2(g) at
constant temperature is increased from 60...

Chemistry, 15.01.2020 04:31 moonlighthowl4537
The pressure on a 200-milliliter sample of co2(g) at
constant temperature is increased from 60 kpa to
120 kpa. what is the new volume of the gas? a) 100 ml b) 300 ml
c) 400 ml d) 600 ml

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Questions


Biology, 01.09.2019 08:00




History, 01.09.2019 08:00

Mathematics, 01.09.2019 08:00



Biology, 01.09.2019 08:00

Social Studies, 01.09.2019 08:00


Computers and Technology, 01.09.2019 08:00

Biology, 01.09.2019 08:00

History, 01.09.2019 08:00



Spanish, 01.09.2019 08:00


Social Studies, 01.09.2019 08:00