Chemistry, 15.01.2020 06:31 santiagobermeo32
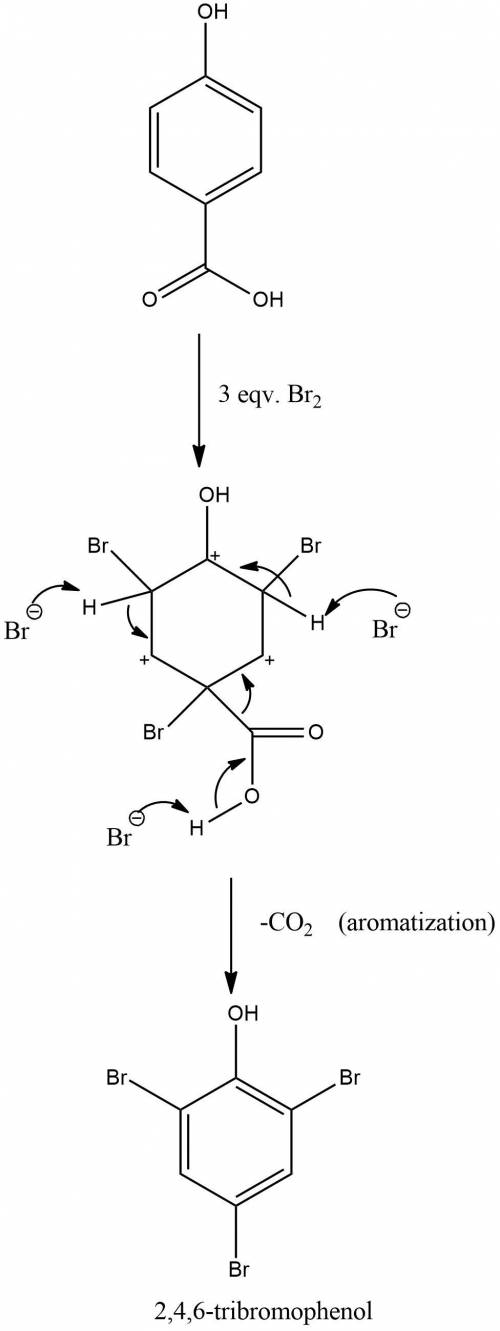
Treatment of p-hydroxybenzoic acid with aqueous bromine leads to the evolution of carbon dioxide and the formation of 2,4,6-tribromophenol. explain.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2


Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
Treatment of p-hydroxybenzoic acid with aqueous bromine leads to the evolution of carbon dioxide and...
Questions

English, 05.03.2021 04:40

SAT, 05.03.2021 04:40



Mathematics, 05.03.2021 04:40

Mathematics, 05.03.2021 04:40

Physics, 05.03.2021 04:40




Mathematics, 05.03.2021 04:40

Mathematics, 05.03.2021 04:40

Social Studies, 05.03.2021 04:40

Mathematics, 05.03.2021 04:40

Mathematics, 05.03.2021 04:40

Mathematics, 05.03.2021 04:40