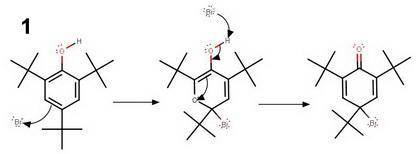
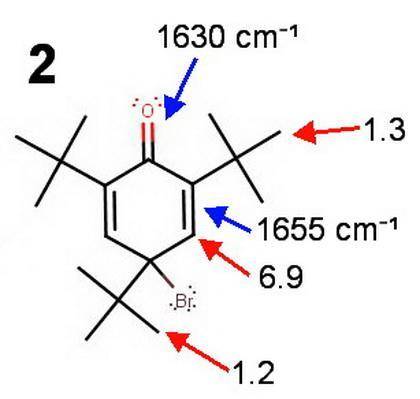
Treatment of 2,4,6-tri-tert-butylphenol with bromine in cold acetic acid gives the compound c18h29bro in quantitative yield. the infrared spectrum of this compound contains absorptions at 1630 and 1655 cm^-1. its 1h nmr spectrum shows only three peaks (all singlets), at δ 1.2, 1.3, and 6.9, in the ratio 9: 18: 2. what is a reasonable structure for the compound?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
Treatment of 2,4,6-tri-tert-butylphenol with bromine in cold acetic acid gives the compound c18h29br...
Questions

History, 29.12.2021 20:00



Business, 29.12.2021 20:00


Mathematics, 29.12.2021 20:00

History, 29.12.2021 20:00

History, 29.12.2021 20:00

Computers and Technology, 29.12.2021 20:00



History, 29.12.2021 20:00

Computers and Technology, 29.12.2021 20:00

Computers and Technology, 29.12.2021 20:00


Mathematics, 29.12.2021 20:00

History, 29.12.2021 20:00


History, 29.12.2021 20:00