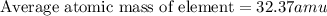Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
You know the right answer?
What is the average atomic mass of chlorine if the mass of one isotope is 34.97 amu and has a p? er...
Questions

Mathematics, 23.01.2020 02:31


Health, 23.01.2020 02:31

History, 23.01.2020 02:31






Biology, 23.01.2020 02:31

History, 23.01.2020 02:31



English, 23.01.2020 02:31


Biology, 23.01.2020 02:31


Biology, 23.01.2020 02:31


Mathematics, 23.01.2020 02:31

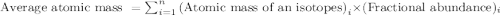
![\text{Average atomic mass of element}=\sum[(34.97\times 0.7577)+(24.23\times 0.2423)]](/tpl/images/0456/3743/30bc9.png)