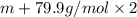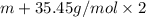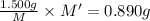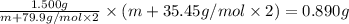Chemistry, 15.01.2020 19:31 whitakers87
An element x has a dibrom ide with the empirical formula xbr2 and a dichloride with the empirical formula xci2. the dibromide is completely converted to the dichloride when it is heated in a stream of chlorine according to the reaction $$xbr2 + cl2 → xci2 + br2$$ when 1.500 g xbr2 is treated, 0.890 g xci2 results. (a) calculate the atomic mass of the element x. (b) by reference to a list of the atomic masses of the elements, identify the element x.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
An element x has a dibrom ide with the empirical formula xbr2 and a dichloride with the empirical fo...
Questions


Mathematics, 12.06.2021 14:00


Mathematics, 12.06.2021 14:00

Mathematics, 12.06.2021 14:00


Mathematics, 12.06.2021 14:00



Mathematics, 12.06.2021 14:00




Computers and Technology, 12.06.2021 14:00



World Languages, 12.06.2021 14:00



Mathematics, 12.06.2021 14:00

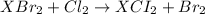
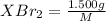 mol
mol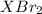 gives 1 mole of
gives 1 mole of 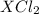 . then
. then 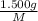 mol of [tex[CBr_2[/tex] will give:
mol of [tex[CBr_2[/tex] will give: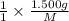 mol=\frac{1.500 g}{M}[/tex] mol[/tex] of
mol=\frac{1.500 g}{M}[/tex] mol[/tex] of