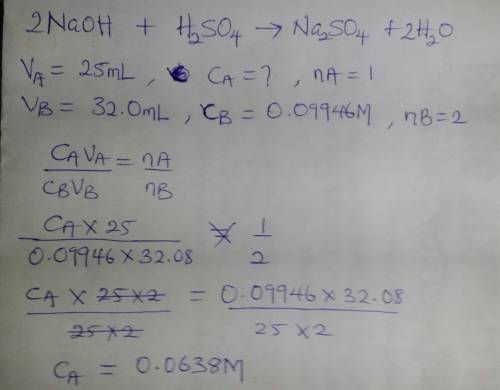Chemistry, 16.01.2020 04:31 risolatziyovudd
Asolution of sulfuric acid is titrated with a solution of sodium hydroxide to the phenolphthalein end point. the sample of sulfuric acid is 25.00 ml. the titration takes 32.08 ml of 0.09946 m sodium hydroxide.
a.) write out a balanced equation for the reaction.
b.) calculate the molarity of the sulfuric acid.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1


Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
Asolution of sulfuric acid is titrated with a solution of sodium hydroxide to the phenolphthalein en...
Questions



English, 23.10.2020 02:01

Chemistry, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01



Mathematics, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01

Advanced Placement (AP), 23.10.2020 02:01


English, 23.10.2020 02:01




History, 23.10.2020 02:01

English, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01