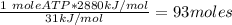Chemistry, 16.01.2020 05:31 JayLiz1737
Ask your teacher referring to the metabolic process below, calculate the maximum number of moles of atp that can be synthesized from adp from the breakdown of one mole of glucose. c6h12o6(s) + 6 o2(g) → 6 co2(g) + 6 h2o(l) δg° = −2880 kj/mol adp + h3po4 → atp + h2o δg° = 31 kj/mol webassign will check your answer for the correct number of significant figures. moles

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 23.06.2019 13:00
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1

Chemistry, 23.06.2019 14:30
The hammering on a train track is often heard twice by workers farther down the track; first as the sound travels through the steel and second as the sound travels through the air. this suggests which graph is true?
Answers: 1

You know the right answer?
Ask your teacher referring to the metabolic process below, calculate the maximum number of moles of...
Questions




Mathematics, 05.03.2021 23:20



Mathematics, 05.03.2021 23:20



Mathematics, 05.03.2021 23:20

Computers and Technology, 05.03.2021 23:20

Mathematics, 05.03.2021 23:20



English, 05.03.2021 23:20


Social Studies, 05.03.2021 23:20



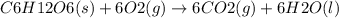 ΔG°=-2880 KJ/mol
ΔG°=-2880 KJ/mol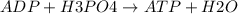 ΔG°=31 KJ/mol
ΔG°=31 KJ/mol