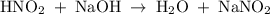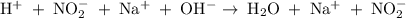Chemistry, 16.01.2020 07:31 elsauceomotho
The net ionic equation for the reaction that occurs during the titration of nitrous acid with sodium hydroxide is (a) hno2 na 1 oh-1 nano2 h2o (b) hno2 naoh na 1 no2-1 h2o (c) h 1 oh-1 h2o (d) hno2 h2o no2-1 h3o 1 (e) hno2 oh-1 no2-1 h2o

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
The net ionic equation for the reaction that occurs during the titration of nitrous acid with sodium...
Questions

Chemistry, 08.06.2021 22:30

Social Studies, 08.06.2021 22:30

English, 08.06.2021 22:30

Mathematics, 08.06.2021 22:30

Health, 08.06.2021 22:30



Mathematics, 08.06.2021 22:30

Mathematics, 08.06.2021 22:30


Mathematics, 08.06.2021 22:30

Mathematics, 08.06.2021 22:30



English, 08.06.2021 22:30

Mathematics, 08.06.2021 22:30


English, 08.06.2021 22:30

English, 08.06.2021 22:30

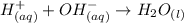
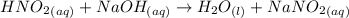
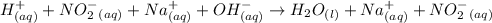
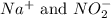 are the spectator ions.
are the spectator ions.
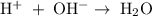 .
.