Abeaker has 0.2 m of na2so4. what will be the concentration of sodium and sulfate ions?
[na+]...

Chemistry, 16.01.2020 17:31 brummy309506
Abeaker has 0.2 m of na2so4. what will be the concentration of sodium and sulfate ions?
[na+] = 0.4 m, [so42-] = 0.2 m
[na+] = 0.2 m, [so42-] = 0.2 m
[na+] = 0.4 m, [so42-] = 0.4 m
[na+] = 0.2 m, [so42-] = 0.4 m

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
Questions

Mathematics, 10.10.2019 09:50

Biology, 10.10.2019 09:50

Chemistry, 10.10.2019 09:50

Mathematics, 10.10.2019 09:50

Health, 10.10.2019 09:50



Mathematics, 10.10.2019 09:50


Mathematics, 10.10.2019 09:50

English, 10.10.2019 09:50


History, 10.10.2019 09:50

Computers and Technology, 10.10.2019 09:50

History, 10.10.2019 09:50

Health, 10.10.2019 09:50

Computers and Technology, 10.10.2019 09:50

Mathematics, 10.10.2019 09:50


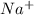 ] = 0.4 M, [
] = 0.4 M, [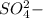 ] = 0.2 M
] = 0.2 M

