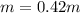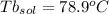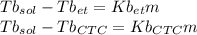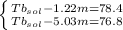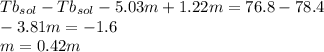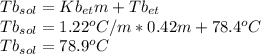Chemistry, 16.01.2020 17:31 naomicervero
Imagine two solutions with the same concentration and the same boiling point, but one has ethanol as the solvent and the other has carbon tetrachloride as the solvent. determine that molal concentration, m (or b ), and boiling point, tb. given: ethanolnormal boiling point: 78.4kb: 1.22ccl4normal boiling point: 76.8kb: 5.03

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
Imagine two solutions with the same concentration and the same boiling point, but one has ethanol as...
Questions



Mathematics, 15.07.2019 18:30





Mathematics, 15.07.2019 18:30

History, 15.07.2019 18:30

History, 15.07.2019 18:30


English, 15.07.2019 18:30



Advanced Placement (AP), 15.07.2019 18:30



Mathematics, 15.07.2019 18:30


English, 15.07.2019 18:30