
Suppose we have a 12.2 l sample containing 0.50 mol oxygen gas at a pressure of 1 atm and a temperature of 25 degrees celcius. if all this oxygen were converted to ozone at the same temperature and pressure, what would be the volume of the ozone? balanced equation: 3o2 → 2o3a. 6.5lb. 2.8lc. 8.1ld. 7.4l

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Suppose we have a 12.2 l sample containing 0.50 mol oxygen gas at a pressure of 1 atm and a temperat...
Questions


Mathematics, 27.05.2021 20:20


Mathematics, 27.05.2021 20:20

Mathematics, 27.05.2021 20:20

Chemistry, 27.05.2021 20:20


Mathematics, 27.05.2021 20:20

Mathematics, 27.05.2021 20:20

English, 27.05.2021 20:20


Mathematics, 27.05.2021 20:20


Mathematics, 27.05.2021 20:20

History, 27.05.2021 20:20

Mathematics, 27.05.2021 20:20


Physics, 27.05.2021 20:20


Mathematics, 27.05.2021 20:20

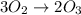
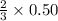 moles of ozone
moles of ozone

