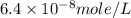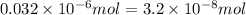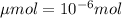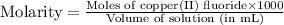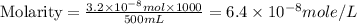Chemistry, 16.01.2020 20:31 starfox5454
Achemist prepares a solution of copper(ii) fluoride (cuf2) by measuring out 0.032 µmol of copper(ii) fluoride into a 500 ml volumetric flask and filling the flask to the mark with water. calculate the concentration in mol/l of the chemist's copper(ii) fluoride solution.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1
You know the right answer?
Achemist prepares a solution of copper(ii) fluoride (cuf2) by measuring out 0.032 µmol of copper(ii)...
Questions

Health, 09.07.2019 17:00

Biology, 09.07.2019 17:00


Biology, 09.07.2019 17:00



Mathematics, 09.07.2019 17:00


History, 09.07.2019 17:00




Social Studies, 09.07.2019 17:00






Mathematics, 09.07.2019 17:00

Social Studies, 09.07.2019 17:00