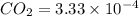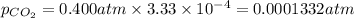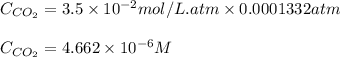Chemistry, 16.01.2020 22:31 deasiamonay14
Calculate the solubility of carbon dioxide in water at an atmospheric pressure of 0.400 atm (a typical value at high altitude).atmospheric gas mole fraction kh mol/(l*atm)n2 7.81 x 10-1 6.70 x 10-4o2 2.10 x 10-1 1.30 x 10-3ar 9.34 x 10-3 1.40 x 10-3co2 3.33 x 10-4 3.50 x 10-2ch4 2.00 x 10-6 1.40 x 10-3h2 5.00 x 10-7 7.80 x 10-4

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1
You know the right answer?
Calculate the solubility of carbon dioxide in water at an atmospheric pressure of 0.400 atm (a typic...
Questions




Chemistry, 12.04.2021 21:10


Biology, 12.04.2021 21:10

Mathematics, 12.04.2021 21:10




Biology, 12.04.2021 21:10


World Languages, 12.04.2021 21:10

English, 12.04.2021 21:10



Mathematics, 12.04.2021 21:10

Mathematics, 12.04.2021 21:10

English, 12.04.2021 21:10

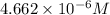 .
.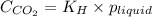
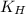 = Henry's constant =
= Henry's constant = 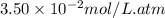
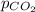 = partial pressure of carbonated drink
= partial pressure of carbonated drink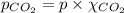
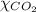 = mole fraction of
= mole fraction of