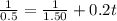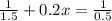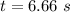Chemistry, 16.01.2020 23:31 jayjinks976
For the simple decomposition reactionab(g)→ a(g) + b(g)rate =k[ab]2 and k=0.2 l/mol*s . how long will it takefor [ab] to reach 1/3 of its initial concentration 1.50 mol/l?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
For the simple decomposition reactionab(g)→ a(g) + b(g)rate =k[ab]2 and k=0.2 l/mol*s . how long wil...
Questions




English, 30.05.2021 04:20

English, 30.05.2021 04:20

English, 30.05.2021 04:20

Biology, 30.05.2021 04:20


Mathematics, 30.05.2021 04:20









Mathematics, 30.05.2021 04:20

French, 30.05.2021 04:20

Mathematics, 30.05.2021 04:20

![Rate = k[AB]^2](/tpl/images/0458/3241/7f1de.png)
![\frac{1}{[A_t]} = \frac{1}{[A]_0}+kt](/tpl/images/0458/3241/f2ee3.png)
![[A_0]](/tpl/images/0458/3241/9a686.png) is the initial concentration = 1.50 mol/L
is the initial concentration = 1.50 mol/L![[A_t]](/tpl/images/0458/3241/5262c.png) is the final concentration = 1/3 of initial concentration =
is the final concentration = 1/3 of initial concentration = 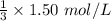 = 0.5 mol/L
= 0.5 mol/L