
Chemistry, 17.01.2020 00:31 pineapplepizaaaaa
Which of the following statements about standard reduction potential and spontaneity are true?
choose one or more:
a. a reaction with a negative gibbs standard free energy is thermodynamically spontaneous under standard conditions.
b. a coupled redox reaction with a negative standard reduction potential is thermodynamically spontaneous.
c. a coupled redox reaction with a positive standard reduction potential îeââ² is thermodynamically spontaneous.
d. a reaction with a positive gibbs standard free energy is thermodynamically spontaneous under standard conditions.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
Which of the following statements about standard reduction potential and spontaneity are true?
Questions


Biology, 30.10.2020 05:00

Arts, 30.10.2020 05:00


English, 30.10.2020 05:10

Geography, 30.10.2020 05:10


English, 30.10.2020 05:10



Physics, 30.10.2020 05:10


Mathematics, 30.10.2020 05:10


Mathematics, 30.10.2020 05:10


Biology, 30.10.2020 05:10

English, 30.10.2020 05:10

History, 30.10.2020 05:10

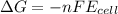 (1)
(1) 

