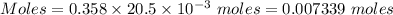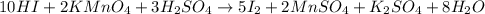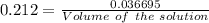Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1


Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
How many milliliters of a 0.212 m hi solution are needed to reduce 20.5 ml of a 0.358 m kmno4 soluti...
Questions

Computers and Technology, 06.03.2021 04:30



English, 06.03.2021 04:30


Biology, 06.03.2021 04:30


Advanced Placement (AP), 06.03.2021 04:30

Mathematics, 06.03.2021 04:30


Advanced Placement (AP), 06.03.2021 04:30



Social Studies, 06.03.2021 04:30

Computers and Technology, 06.03.2021 04:30





Mathematics, 06.03.2021 04:30

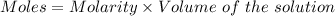
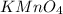 :
: