
Chemistry, 10.10.2019 19:30 LikeIke2859
According to the equation 2na + 2h2o mc012-1.jpg 2naoh+h2, what mass of na is required to yield 22.4 l of h2 at stp? (the atomic mass of na is 22.99 u.)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1
You know the right answer?
According to the equation 2na + 2h2o mc012-1.jpg 2naoh+h2, what mass of na is required to yield 22.4...
Questions


Mathematics, 25.03.2021 18:10


Law, 25.03.2021 18:10

Geography, 25.03.2021 18:10

Chemistry, 25.03.2021 18:10






Physics, 25.03.2021 18:10


Chemistry, 25.03.2021 18:10




Mathematics, 25.03.2021 18:10


History, 25.03.2021 18:10


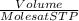
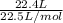
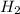 : Na is 1 : 2
: Na is 1 : 2

