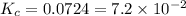Chemistry, 18.01.2020 00:31 honeylebling
Mercury(ii) oxide decomposes to form mercury and oxygen, like this: (s)(l)(g) at a certain temperature, a chemist finds that a reaction vessel containing a mixture of mercury(ii) oxide, mercury, and oxygen at equilibrium has the following composition:

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
Mercury(ii) oxide decomposes to form mercury and oxygen, like this: (s)(l)(g) at a certain temperat...
Questions

Biology, 10.01.2021 01:00

Geography, 10.01.2021 01:00




Mathematics, 10.01.2021 01:00





English, 10.01.2021 01:00

SAT, 10.01.2021 01:00

Health, 10.01.2021 01:00



Mathematics, 10.01.2021 01:00

English, 10.01.2021 01:00

Mathematics, 10.01.2021 01:00

English, 10.01.2021 01:00

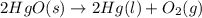
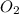 22.7 g
22.7 g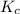 for this reaction. Round your answer to 2 significant digits.
for this reaction. Round your answer to 2 significant digits.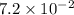
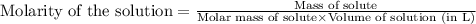
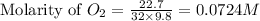
![K_c=[O_2]](/tpl/images/0460/0182/99fa8.png)