

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1

Chemistry, 23.06.2019 14:00
If you fill your car tire to a pressure of 32 psi (pounds per square inch) on a hot summer day when the temperature is 35°c (95°f), what is the pressure (in psi) on a cold winter day when the temperature is -15°c (5°f)? assume no gas leaks out between measurements and the volume of the tire does not change.
Answers: 1
You know the right answer?
For h3po4, ka1 = 7.3 x 10^-3, ka2 = 6.2 x 10^-6, and ka3 = 4.8 x 10^-13. a 0.10 m aqueous solution o...
Questions

Mathematics, 09.07.2019 13:00


Mathematics, 09.07.2019 13:00


Arts, 09.07.2019 13:00


Mathematics, 09.07.2019 13:00

Arts, 09.07.2019 13:00

History, 09.07.2019 13:00

Mathematics, 09.07.2019 13:00


Mathematics, 09.07.2019 13:00

Mathematics, 09.07.2019 13:00







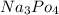 therefore would be "strongly basic."
therefore would be "strongly basic."

