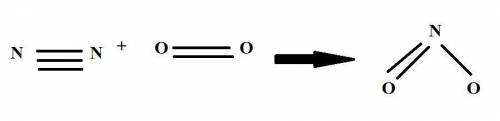
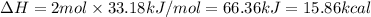Nitrogen in air reacts at high temperature to form no2 according to the reaction:
n2 +...

Chemistry, 18.01.2020 04:31 dchirunga23
Nitrogen in air reacts at high temperature to form no2 according to the reaction:
n2 + 2 o2 ? 2 no2
draw structures for the reactants and products indicating the number of single, double and triple bonds.
determine the ? h for the reaction using table 7.1.
[hint: one no2 has one [n-o] bond and one [n=o] bond]
a.
+115 kcal
b.
-78 kcal
c.
+78 kcal
d.
+7 kcal
e.
none of these

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

You know the right answer?
Questions



History, 23.10.2020 03:01

Mathematics, 23.10.2020 03:01


Mathematics, 23.10.2020 03:01



Mathematics, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01


Mathematics, 23.10.2020 04:01

Computers and Technology, 23.10.2020 04:01



English, 23.10.2020 04:01


Social Studies, 23.10.2020 04:01


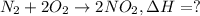
![\Delta H=[2 mol\times \Delta H_{f,NO_2}]-[1 mol\times \Delta H_{f,N_2}-2 mol\times \Delta H_{f,O_2}]](/tpl/images/0460/4581/de02f.png)
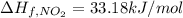
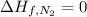 (pure element)
(pure element)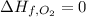 (pure element )
(pure element )