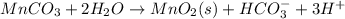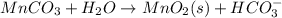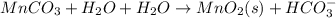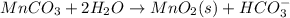Write a half-reaction for the oxidation of the manganese in mnco3(s) to mno2(s) in neutral groundwater where the carbonate-containing species in the product is hco3–(aq). add h2o and h+ to balance the h and o atoms in the equation. do not add electrons; you may leave the half-reaction unbalanced with respect to charge

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3


Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
Write a half-reaction for the oxidation of the manganese in mnco3(s) to mno2(s) in neutral groundwat...
Questions

History, 06.05.2020 20:08



Biology, 06.05.2020 20:08

Mathematics, 06.05.2020 20:08

Mathematics, 06.05.2020 20:08


Mathematics, 06.05.2020 20:09







History, 06.05.2020 20:09





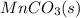 to
to 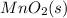 .:
.: