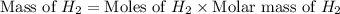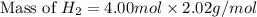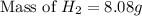Chemistry, 18.01.2020 06:31 bryantjorell
The molar mass of hydrogen (h₂) is 2.02 g/mol. a sample contains 4.00 mol of h₂. what is the mass, in grams, of this sample?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
The molar mass of hydrogen (h₂) is 2.02 g/mol. a sample contains 4.00 mol of h₂. what is the mass, i...
Questions


Mathematics, 02.07.2019 00:30





Mathematics, 02.07.2019 00:30

Mathematics, 02.07.2019 00:30



Mathematics, 02.07.2019 00:30

Mathematics, 02.07.2019 00:30

Mathematics, 02.07.2019 00:30



History, 02.07.2019 00:30

Mathematics, 02.07.2019 00:30


Mathematics, 02.07.2019 00:30

Mathematics, 02.07.2019 00:30

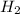 = 2.02 g/mol
= 2.02 g/mol