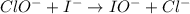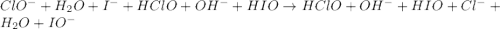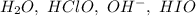Consider this mechanism:
step 1: clo-(aq) + h2o(l) hclo(aq) + oh-(aq) (fast)
step 2:...

Consider this mechanism:
step 1: clo-(aq) + h2o(l) hclo(aq) + oh-(aq) (fast)
step 2: i-(aq) + hclo(aq) hio(aq) + cl-(aq) (slow)
step 3: oh-(aq) + hio(aq) h2o(l) + io-(aq) (fast)
what is the overall reaction?
a.
hclo (aq) + h2o (l) oh- (aq) + cl- (aq)
b.
i- (aq) + hclo (aq) hio (aq) + cl- (aq)
c.
oh- (aq) +hio (aq) h2o (l) + io- (aq)
d.
clo- (aq) + i- (aq) cl- (aq) + io- (aq)
e.
clo- (aq) + h2o(l) hclo(aq) + oh- (aq)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2


Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
Questions

Mathematics, 31.03.2020 18:14

Social Studies, 31.03.2020 18:14


Social Studies, 31.03.2020 18:14



Mathematics, 31.03.2020 18:14




Biology, 31.03.2020 18:14

Social Studies, 31.03.2020 18:14





Biology, 31.03.2020 18:14


Mathematics, 31.03.2020 18:14