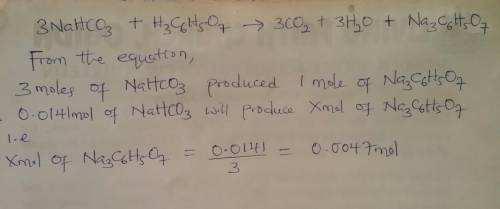when an antacid tablet dissolves in water, the fizz is due to a reaction between sodium hydrogen carbonate (sodium bicarbonate, nahco3) and citric acid (h3c6h5o7).
3 nahco3(aq) + h3c6h5o7(aq) 3 co2(g) + 3 h2o(l) + na3c6h5o7(aq)
how many moles of na3c6h5o7 can be produced if one tablet containing 0.0141 mol of nahco3 is dissolved?
mol

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
when an antacid tablet dissolves in water, the fizz is due to a reaction between sodium hydrogen car...
Questions

History, 05.11.2020 03:30



Biology, 05.11.2020 03:30

Mathematics, 05.11.2020 03:30




Spanish, 05.11.2020 03:30


Biology, 05.11.2020 03:30

English, 05.11.2020 03:30

Chemistry, 05.11.2020 03:30

History, 05.11.2020 03:30


Mathematics, 05.11.2020 03:30