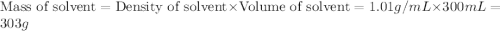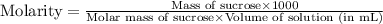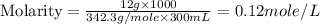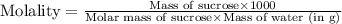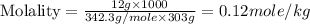Astudent dissolves 12.g of sucrose c12h22o11 in 300.ml of a solvent with a density of 1.01/gml . the student notices that the volume of the solvent does not change when the sucrose dissolves in it. calculate the molarity and molality of the student's solution. round both of your answers to 2 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 09:10
A155.0 9 piece of copper at 182 °c is dropped into 2500 g of water at 23.9 °c. (the specific heat of copper is 0.385 1/9°c.) calculate the final temperature of the mixture. (assume no heat loss to the surroundings.)
Answers: 2

Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 1

Chemistry, 23.06.2019 12:30
0.070g of hydride of carbon occupies 56cm^3 at s.t.p when vaporized and contained 14.29% by mass of hydrogen.what is the formula for the hydrocarbon
Answers: 1
You know the right answer?
Astudent dissolves 12.g of sucrose c12h22o11 in 300.ml of a solvent with a density of 1.01/gml . the...
Questions









Chemistry, 04.12.2021 01:10


Business, 04.12.2021 01:10