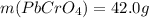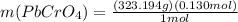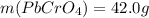Asolution of potassium chromate reacts with a solution of lead(ii) nitrate to produce a yellow precipitate of lead(ii) chromate and a solution of potassium nitrate.
(a) write the balanced chemical equation. (use the lowest possible coefficients. include states-of-matter under the given conditions in your answer.)
(b) starting with 0.130 mol potassium chromate, determine the mass of lead chromate that can be formed.
g


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Asolution of potassium chromate reacts with a solution of lead(ii) nitrate to produce a yellow preci...
Questions

Mathematics, 06.01.2021 22:40



Mathematics, 06.01.2021 22:40



World Languages, 06.01.2021 22:40

English, 06.01.2021 22:40

History, 06.01.2021 22:40

Mathematics, 06.01.2021 22:40


Mathematics, 06.01.2021 22:40





Mathematics, 06.01.2021 22:40


Mathematics, 06.01.2021 22:40