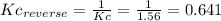Chemistry, 20.01.2020 19:31 naomijefferson22
Balance each of the following examples of heterogeneous equilibria and write each kc expression. then calculate the value of kc for the reverse reaction.
(1) al(s) + naoh(aq) + h2o(l) ⇋ na[al(oh)4](aq) + h2(g) kc for balanced reaction = 11
(2) h2o(l) + so3(g) ⇋ h2so4 (aq) kc for balanced reaction = 0.0123
(3) p4(s) + o2(g) ⇋ p4o6(s) kc for balanced reaction = 1.56

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Balance each of the following examples of heterogeneous equilibria and write each kc expression. the...
Questions

Social Studies, 09.02.2022 09:10

English, 09.02.2022 09:10




Computers and Technology, 09.02.2022 09:20

Social Studies, 09.02.2022 09:20



Computers and Technology, 09.02.2022 09:20

SAT, 09.02.2022 09:20

Mathematics, 09.02.2022 09:20




Mathematics, 09.02.2022 09:20



Mathematics, 09.02.2022 09:20

 + 7 H_2(g)](/tpl/images/0462/9109/35a9e.png)
![Kc=\frac{[Na[Al(OH)_4]]^2*[H_2]^7}{[NaOH]^2}](/tpl/images/0462/9109/5fa1b.png)
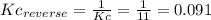
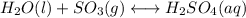
![Kc=\frac{[H_2SO_4]}{[SO_3]^2}](/tpl/images/0462/9109/da917.png)
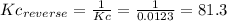
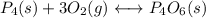
![Kc=\frac{1}{[O_2]^3}](/tpl/images/0462/9109/3ee9b.png)