
Chemistry, 20.01.2020 19:31 lavardamon123
Write the formula of the conjugate base of each acid: hbr, hco3−, and ch3ch2ch2oh. be sure to answer all parts. (note: if a number has been placed as a subscript, the cursor needs to be returned to the main writing line before selecting the superscript.)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
Write the formula of the conjugate base of each acid: hbr, hco3−, and ch3ch2ch2oh. be sure to answe...
Questions

Health, 01.08.2019 04:00

Mathematics, 01.08.2019 04:00


Biology, 01.08.2019 04:00

Biology, 01.08.2019 04:00

Mathematics, 01.08.2019 04:00

History, 01.08.2019 04:00


Biology, 01.08.2019 04:00

Chemistry, 01.08.2019 04:00


Chemistry, 01.08.2019 04:00


Mathematics, 01.08.2019 04:00



Business, 01.08.2019 04:00

Mathematics, 01.08.2019 04:00


Biology, 01.08.2019 04:00

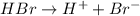
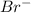 is the conjugate base of HBr.
is the conjugate base of HBr.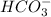
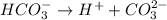
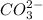 is the conjugate base of
is the conjugate base of 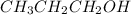
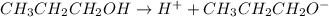
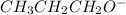 is the conjugate base of
is the conjugate base of 

