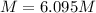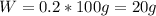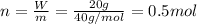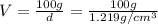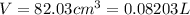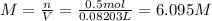Chemistry, 20.01.2020 20:31 madams4450
A20.0%(m/m) solution of naoh (fm 40.00) has a density of 1.219 g/cm'. calculate the solution's molarity. (6.10 m) 2. * 10 w= amountiof solute d- density of solution motanty m= molecular mass ot solute %3d

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
A20.0%(m/m) solution of naoh (fm 40.00) has a density of 1.219 g/cm'. calculate the solution's molar...
Questions


Health, 22.07.2020 05:01

Mathematics, 22.07.2020 05:01



Mathematics, 22.07.2020 05:01


Chemistry, 22.07.2020 05:01






Mathematics, 22.07.2020 05:01


Computers and Technology, 22.07.2020 05:01


Mathematics, 22.07.2020 05:01

Mathematics, 22.07.2020 05:01