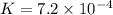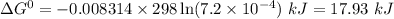Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1


Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
Calculate δg° for the following reaction from the equilibrium constant at the temperature given. hf(...
Questions


English, 05.09.2021 14:20


Mathematics, 05.09.2021 14:20

Chemistry, 05.09.2021 14:20




Biology, 05.09.2021 14:20

English, 05.09.2021 14:20

Computers and Technology, 05.09.2021 14:20


Geography, 05.09.2021 14:20




Biology, 05.09.2021 14:20

Social Studies, 05.09.2021 14:20

Mathematics, 05.09.2021 14:20

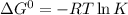
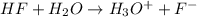
![25^oC=[25+273]K=298K](/tpl/images/0462/9739/df1f6.png)