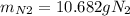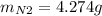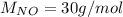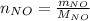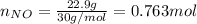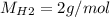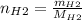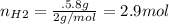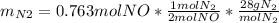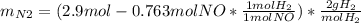Chemistry, 20.01.2020 23:31 jewlbug4358
For the following reaction, 22.9 grams of nitrogen monoxide are allowed to react with 5.80 grams of hydrogen gas. nitrogen monoxide (g) + hydrogen (g)> nitrogen (g) + water (1) what is the maximum amount of nitrogen gas that can be formed? what is the formula for the limiting reagent? grams what amount of the excess reagent remains after the reaction is complete? grams

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
For the following reaction, 22.9 grams of nitrogen monoxide are allowed to react with 5.80 grams of...
Questions

Mathematics, 22.06.2021 17:00

Mathematics, 22.06.2021 17:00




History, 22.06.2021 17:00

Mathematics, 22.06.2021 17:00


History, 22.06.2021 17:00



World Languages, 22.06.2021 17:00


Mathematics, 22.06.2021 17:00





Mathematics, 22.06.2021 17:00