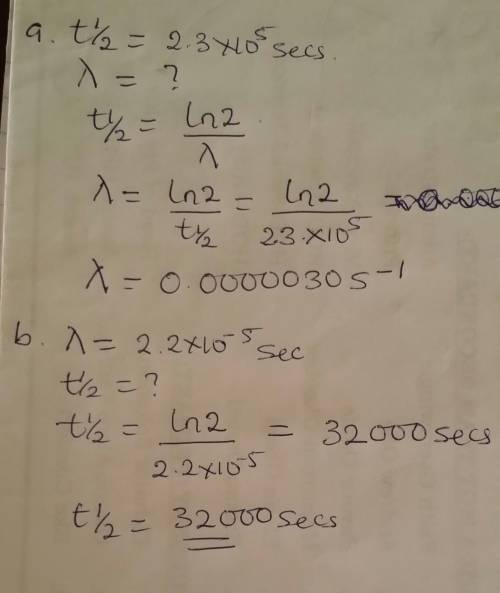Chemistry, 20.01.2020 23:31 ayoismeisjuam
The gas-phase decomposition of so2cl2, so2cl2(g)→so2(g)+cl2(g), is first order in so2cl2. at 600 k the half-life for this process is 2.3×105s.
part a
what is the rate constant at this temperature?
express your answer using two significant figures.
part b
at 320 ∘c the rate constant is 2.2×10−5s−1. what is the half-life at this temperature?
express your answer using two significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3


Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1
You know the right answer?
The gas-phase decomposition of so2cl2, so2cl2(g)→so2(g)+cl2(g), is first order in so2cl2. at 600 k t...
Questions





Mathematics, 17.11.2019 07:31

Mathematics, 17.11.2019 07:31




SAT, 17.11.2019 07:31





Mathematics, 17.11.2019 07:31

English, 17.11.2019 07:31

Mathematics, 17.11.2019 07:31


English, 17.11.2019 07:31

Mathematics, 17.11.2019 07:31