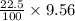Chemistry, 21.01.2020 01:31 pineapplepizaaaaa
The dissociation of calcium carbonate has an equilibrium constant of kp= 1.20 at 800°c. caco3(s) ⇋ cao(s) + co2(g)
a.) what is the kc for the reaction?
b.) if you place 20.0 g of caco3 in a 2.50 l container at 800°c, what is the pressure of co2 in the container?
c.) what percentage of the original 20.0 g sample of caco3 remains undecomposed at equilibrium?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
The dissociation of calcium carbonate has an equilibrium constant of kp= 1.20 at 800°c. caco3(s) ⇋ c...
Questions



Mathematics, 03.03.2021 19:20





Mathematics, 03.03.2021 19:20

English, 03.03.2021 19:20

History, 03.03.2021 19:20

Mathematics, 03.03.2021 19:20

Mathematics, 03.03.2021 19:20


Spanish, 03.03.2021 19:20






 and
and  is as follows.
is as follows.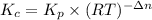
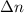 = 1
= 1
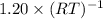


![p[CO_{2}] = K_{p}](/tpl/images/0463/2934/4d1a8.png) = 1.20
= 1.20
 in the container is 1.20.
in the container is 1.20.![K_{c} = \frac{[CaO][CO_{2}]}{[CaCO_{3}]}](/tpl/images/0463/2934/031cb.png)