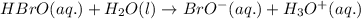Chemistry, 21.01.2020 03:31 holaadios222lol
Problem page fill in the left side of this equilibrium constant equation for the reaction of hypobromous acid with water.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
Problem page fill in the left side of this equilibrium constant equation for the reaction of hypobro...
Questions

Mathematics, 19.03.2020 09:16

Chemistry, 19.03.2020 09:17


Social Studies, 19.03.2020 09:17


History, 19.03.2020 09:17







Mathematics, 19.03.2020 09:18

Mathematics, 19.03.2020 09:18

Biology, 19.03.2020 09:18

Mathematics, 19.03.2020 09:18



English, 19.03.2020 09:18

![K_a=\frac{[BrO^-][H_3O^+]}{[HBrO]}](/tpl/images/0463/4696/6706c.png)
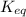
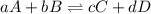
![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0463/4696/9c8b0.png)