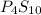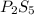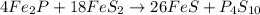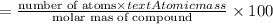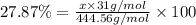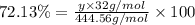Ferrophosphorus (fe2p) reacts with pyrite (fes2) producing iron(ii) sulfide and a compound that is 27.87% p and 72.13% s by mass and has a molar mass of 444.56 g/mol.
a. determine the empirical and molecular formulas of this compound.
b. empirical formula: molecular formula:
c. write a balanced chemical equation for this reaction. do not include phases.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Ferrophosphorus (fe2p) reacts with pyrite (fes2) producing iron(ii) sulfide and a compound that is 2...
Questions

Mathematics, 31.05.2020 07:57

Mathematics, 31.05.2020 07:57

Mathematics, 31.05.2020 07:57

Chemistry, 31.05.2020 07:57

Mathematics, 31.05.2020 07:57

Computers and Technology, 31.05.2020 07:57


Business, 31.05.2020 07:57

Mathematics, 31.05.2020 07:57

Mathematics, 31.05.2020 07:57



Mathematics, 31.05.2020 07:57

Mathematics, 31.05.2020 07:57

Mathematics, 31.05.2020 07:57


Mathematics, 31.05.2020 07:57

Law, 31.05.2020 07:57