
Reaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. the gas was collected by water displacement at 22 degree c. the barometric pressure in the lab was 746 mm hg. the vapor pressure of water at 22 degree c is 19.8 mm hg. use dalton's law to calculate the partial pressure of hydrogen gas in the gas-collecting tube. use the combined gas law to calculate the volume of hydrogen at stp. what is the theoretical number of moles of hydrogen that can be produced from 0.028g of mg?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1
You know the right answer?
Reaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. th...
Questions

History, 06.11.2021 05:40

English, 06.11.2021 05:40

Mathematics, 06.11.2021 05:40

History, 06.11.2021 05:40












English, 06.11.2021 05:40

Arts, 06.11.2021 05:40


Mathematics, 06.11.2021 05:40

Biology, 06.11.2021 05:40

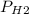 "
"
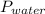
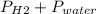
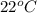 and 726.2 mm Hg and than convert it to STP conditions.
and 726.2 mm Hg and than convert it to STP conditions.
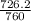
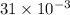 Litre
Litre
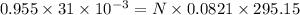
 moles
moles
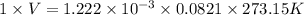
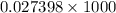 (as 1 L = 1000 ml)
(as 1 L = 1000 ml)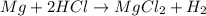
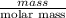
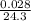
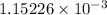 moles
moles


