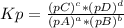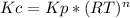Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

You know the right answer?
Ammonium hydrosulfide decomposes to ammonia and hydrogen sulfide gases. find kc for the decompositio...
Questions



Mathematics, 12.11.2020 02:50

Mathematics, 12.11.2020 02:50

Mathematics, 12.11.2020 02:50

Computers and Technology, 12.11.2020 02:50

Social Studies, 12.11.2020 02:50



Biology, 12.11.2020 02:50

Mathematics, 12.11.2020 02:50

History, 12.11.2020 02:50

English, 12.11.2020 02:50



Advanced Placement (AP), 12.11.2020 02:50

Physics, 12.11.2020 02:50



![Kc = \frac{[C]^c*[D]^d}{[A]^a*[B]^b}](/tpl/images/0463/5599/833e7.png)