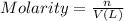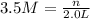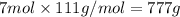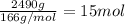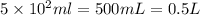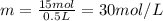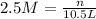Chemistry, 09.10.2019 21:40 arthurcasella
1. how many grams of cacl2 would be required to produce a 3.5 m (molar) solution with a volume of 2.0 l? . 2. what is the molarity of a 5.00 x 102 ml solution containing 2490 g of ki? . 3. how many moles of lif would be required to produce a 2.5 m solution with a volume of 10.5 l? .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
1. how many grams of cacl2 would be required to produce a 3.5 m (molar) solution with a volume of 2....
Questions



Chemistry, 15.09.2021 14:00

Mathematics, 15.09.2021 14:00


English, 15.09.2021 14:00

Mathematics, 15.09.2021 14:00

Biology, 15.09.2021 14:00

Biology, 15.09.2021 14:00

History, 15.09.2021 14:00

Mathematics, 15.09.2021 14:00

English, 15.09.2021 14:00

Chemistry, 15.09.2021 14:00

Chemistry, 15.09.2021 14:00


Mathematics, 15.09.2021 14:00

Computers and Technology, 15.09.2021 14:00

Chemistry, 15.09.2021 14:00


Mathematics, 15.09.2021 14:00